AT2 migrating along the alveolar septum watch
Current research
Defining the molecular and cellular mechanisms of alveolar repair in a live mammal
Recent studies have revealed that a previously uncharacterized cell state is generated transiently during the differentiation of AT2 to AT1 cells, and from airway stem/progenitor cells into AT1 cells. These “intermediate progenitor cells” contribute to repair but may also play a role in disease. Our lab is pioneering new 3-photon time-lapse intravital imaging in the live mammalian lung through a permanent lung window in order to better understand the dynamic behavior and functional role of intermediate progenitor cells during repair in different injury models. In parallel, we are using single cell multi-omics to define gene regulatory pathways that drive intermediate progenitor cell fate and function. Together, this project will define the in vivo molecular and cellular mechanisms that regulate the emergence and differentiation of intermediate progenitor cells into AT1 cells in response to different types of lung injury. With these tools in hand we are excited to explore the dynamic behaviors and interactions of other key cell types implicated in chronic lung disease including immune cells and fibroblasts. We are also keen to explore how and why these responses and lung regeneration in general changes with age.
Tissue regeneration is the process of renewal or growth to replace tissue that is damaged through injury or disease. Failure to correctly regenerate tissue can lead to diseases, such as cancer and fibrosis. Despite improvements in our understanding of stem cell biology, the cellular and molecular mechanisms that underlie tissue regeneration are poorly understood.
Our research focuses on the molecular mechanisms and cellular behaviors that drive dynamic cell transitions during mammalian stem cell-based regeneration, and how de-regulation of these pathways contributes to the pathophysiology of lung diseases.
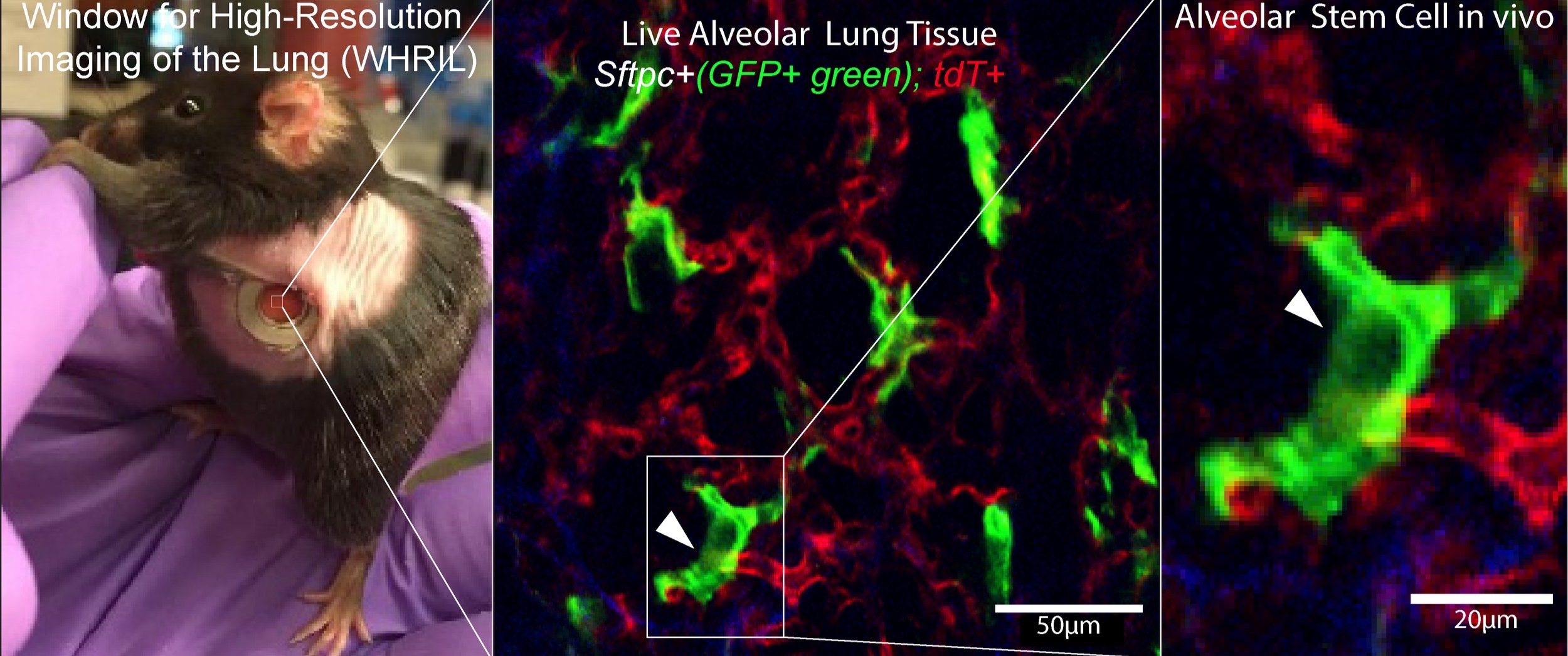
In vivo live imaging via the permanent lung window system allows tracking of individual AT2 stem cells (GFP+; green) in real time over extended periods (panel A re adapted form Entenberg et al, 2018)
Intermediate progenitor cell (IPC) transitions during alveolar injury and repair.
Dissecting stem cell – niche interactions required for alveolar homeostasis and repair
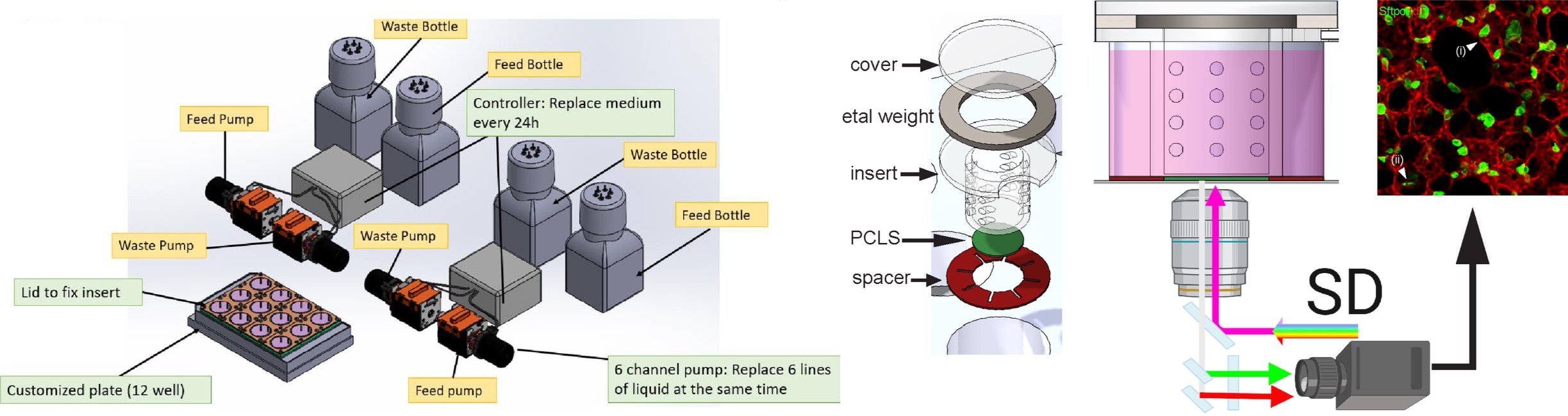
The AT2 stem cell niche is comprised of different cell types including epithelial, mesenchymal, and immune cells. Each cell type in the niche has an important roles in conferring and maintaining AT2 stemness, and in regulating their cellular behavior in response to injury. Despite this, the direct, functional dependency of AT2 cells on individual components of the stem cell niche has never been tested and observed in real time. In collaboration with Dr David Entenberg at Einstein College of Medicine, we are developing a single cell laser ablation approach compatible with the lung window imaging system described above that will enable us to test these dependencies in vivo in the living, breathing mammalian lung. High resolution spatial transcriptomics will allow us to identify potential regulators of these cell-cell interactions in intact lung tissue. In parallel, we have developed a Lung Explant Imaging System (LEIS), a custom-designed chamber for ex vivo culture of intact lung tissue slices that will allow us to manipulate stem cell niche signaling while observing the AT2 stem cell response in real time.
Schematic of in-vivo single cell laser ablation through the permanent lung window
Schematic of LEIS system for ex-vivo PCLS live imaging
Towards new therapies for lung disease
Our research aims to uncover molecular regulators that either promote or perturb lung regeneration. We are interested in exploring whether any of these regulators may be suitable candidates for therapeutic development in the context of chronic lung disease. To this end, we have adapted our LEIS system for ex vivo human PCLS in order to deliver small molecules with precision and reproducibility to human lung tissue. We have previously shown that can treat the human lung slices with a fibrotic cocktail as a model for early stage fibrosis, which we can then use to assess potential drug candidates. Our platform is compatible with the co-culture of other cells and microbiota, allowing us to probe a plethora of different lung disease paradigms. We are also interested in exploring the utility of a human organoid-based system with a fluorescent read-out assay for drug screening. Ultimately, we hope that our research will uncover new druggable targets for the treatment of lung diseases.